Subvenite Titration
Simplified
SUBVENITE Starter Kits are OWP Pharmaceuticals’ generic alternative (AB rated) to Lamictal® (lamotrigine) Starter Kits*

For What Conditions is SUBVENITE Indicated?
SUBVENITE (lamotrigine tablets, USP) are indicated for:
Epilepsy—adjunctive therapy in patients aged 2 years and older:
- partial-onset seizures.
- primary generalized tonic-clonic seizures.
- generalized seizures of Lennox-Gastaut syndrome.
Epilepsy—monotherapy in patients aged 16 years and older: Conversion to monotherapy in patients with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug.
Bipolar disorder: Maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes in patients treated for acute mood episodes with standard therapy.

For What Conditions is Lamotrigine Indicated?
SUBVENITE (lamotrigine tablets, USP) are indicated for:
Epilepsy—adjunctive therapy in patients aged 2 years and older:
- partial-onset seizures.
- primary generalized tonic-clonic seizures.
- generalized seizures of Lennox-Gastaut syndrome.
Epilepsy—monotherapy in patients aged 16 years and older: Conversion to monotherapy in patients with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug.
Bipolar disorder: Maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes in patients treated for acute mood episodes with standard therapy.
Why Choose SUBVENITE Starter Kits?
Initiate Treatment in a Structured Way
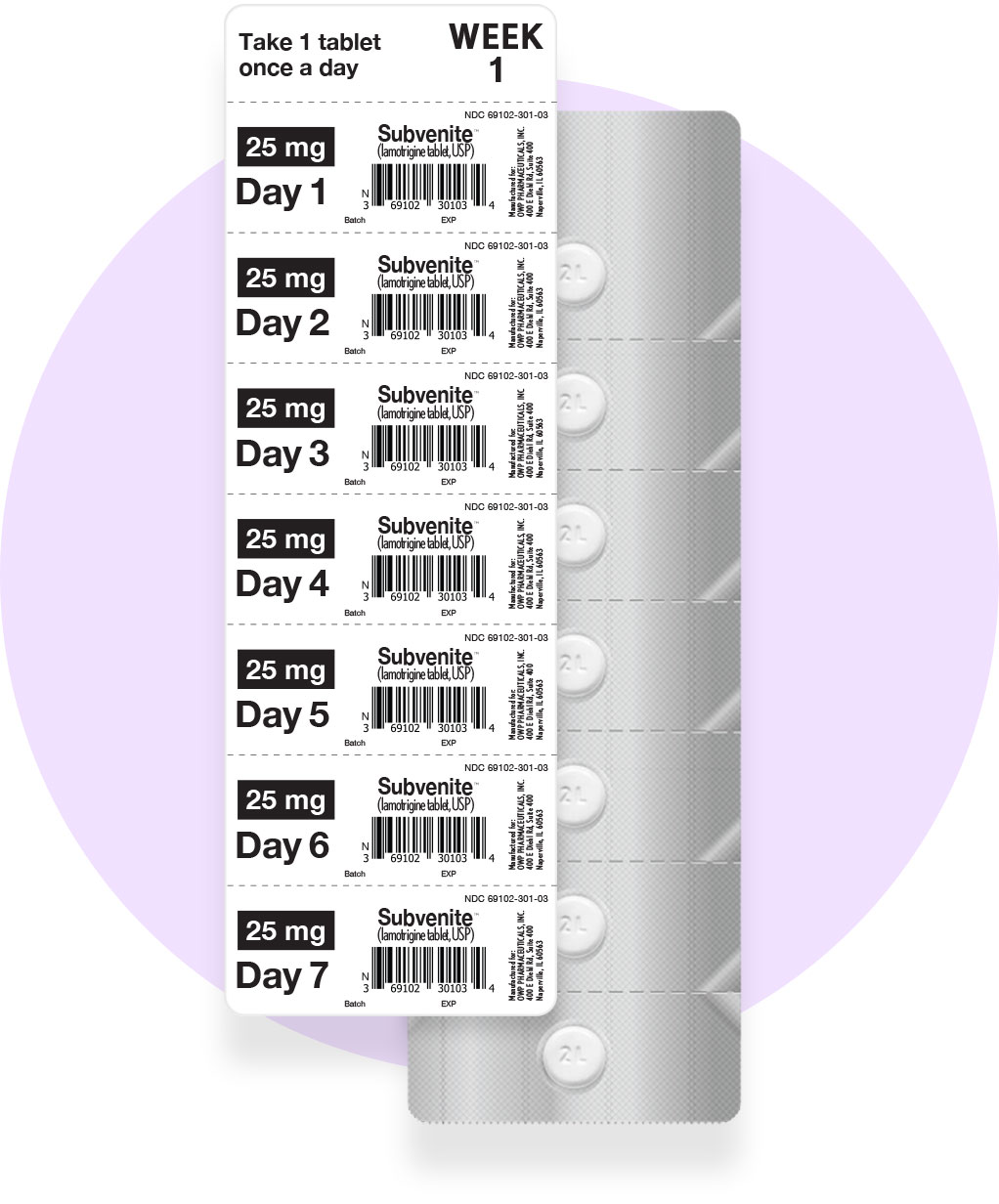
OWP offers three Starter Kits (Orange, Blue, and Green), each based on FDA-approved 5-week titration schedules, for patients initiating lamotrigine therapy. These Kits help healthcare providers titrate their patients appropriately to the therapeutic dose, as supported by clinical studies.
SUBVENITE Starter Kits may be prescribed “DAW-1” (Dispense As Written – or appropriate language required by your state) to ensure the SUBVENITE Starter Kit is received.
A single prescription written or e-prescribed “DAW-1” for the SUBVENITE Starter Kit helps prescribers titrate their patients appropriately to the therapeutic dose, and may help reduce patient confusion and improve compliance. 2,3,4
OWP offers three Starter Kits (Orange, Blue, and Green), each based on FDA-approved 5-week titration schedules, for patients initiating lamotrigine therapy. These Kits help healthcare providers titrate their patients appropriately to the therapeutic dose, as supported by clinical studies.
SUBVENITE Starter Kits may be prescribed “DAW-1” (Dispense As Written – or appropriate language required by your state) to ensure the SUBVENITE Starter Kit is received.
A single prescription written or e-prescribed “DAW-1” for the SUBVENITE Starter Kit helps prescribers titrate their patients appropriately to the therapeutic dose, and may help reduce patient confusion and improve compliance. 2,3,4

Orange Starter Kit: Total 49 Tablets
(42 X 25mg) (7 X 100mg)
NDC 69102-300-01

Blue Starter Kit: Total 35 Tablets
(35 X 25mg)
NDC 69102-306-01

Green Starter Kit: Total 98 Tablets
(84 X 25mg) (14 X 100mg)
NDC 69102-312-01
Convenient Savings Program for Cost Affordability
Pay as little as $5 for any SUBVENITE Starter Kit. With the SUBVENITE Patient Savings Card, patients can receive SUBVENITE Starter Kits at a significantly lower cost than Lamictal (lamotrigine) tablets.*†1
An Opportunity to Help Others
A portion of the profits from the sale of SUBVENITE Starter Kits helps support the humanitarian work of ROW Global Health ↗, an organization dedicated to help bring training, diagnostics, and treatment to under-resourced communities around the world.

Talk to your prescribing healthcare provider to see if a SUBVENITE Starter Kit may be right for you
If you are a patient with a prescription for a SUBVENITE Starter Kit, it can be purchased through your local pharmacy. You may also call 800-273-6729 for assistance in finding a pharmacy to fill your prescription.
If you are a patient currently taking lamotrigine or you are interested in learning more about SUBVENITE Starter Kits, please visit the SUBVENITE Starter Kit Patient Page.
*Lamictal® is a registered trademark of GlaxoSmithKline, Inc. †Terms and conditions apply; see subvenitestarterkits.com/savings-program 1https://www.goodrx.com/lamictal?dosage=49-tablets-of-25mg-and-100mg-orange&form=kit&label_override=Lamictal&quantity=1 2Conn VS, et. al. Current Medical Research and Opinion. 2015;31:1;145-160. 3Gutierrez PM, et. al. Journal of Psychiatric Practice. 2017;23:5;320–327. 4Peterson GM, et. al. Epilepsia. 1984;25:4;412-417.
For general questions or inquiries please contact us at: info@owppharma.com
For SUBVENITE Starter Kit questions please contact us at: medinfo@owppharma.com
To report safety-related concerns please contact us at: safety@owppharma.com
OWOS1062V8 3/25
